Atlas Treatment Facility Survey FAQs
Please find answers to frequently asked questions about the survey here.
About Atlas Treatment Facility Survey (TFS)
What is the Atlas Treatment Facility Survey?
The Atlas Treatment Facility Survey is a data collection instrument that enables providers to report on the services, processes, and structures at their treatment site or clinic (“facility”). Shatterproof collects this information to display in facility profiles on TreatmentAtlas.org. By submitting your data, you are ensuring that up-to-date information on your site or clinic is available for people to use when seeking addiction treatment on Treatment Atlas.
How do I complete the Atlas Treatment Facility Survey?
The Atlas Treatment Facility Survey is available to all addiction treatment providers in Atlas states. You will receive an email from atlas@email.shatterproof.org that includes a specific survey link for your treatment facility or clinic (or multiple links if you are the primary contact for two or more locations) – please use your specific survey link(s) ONLY. We suggest that the staff most familiar with the services and processes available at your site or clinic complete the survey. You will have the option to save your survey responses and return to the survey at a later time if needed. Your progress will be maintained.
Who at my facility should complete this survey?
We recommend that a staff person most familiar with the treatment services provided by your facility, such as a clinical or program director, complete this survey, but feel free to gather information from several staff members if needed. It is perfectly fine to work with colleagues collaboratively to complete the survey.
Can multiple people complete the survey at the same time?
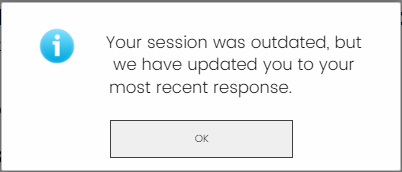
We recommend that only one person from your organization access the online survey at a time. If a second person enters a survey, they may overwrite responses. You will also receive an error message showing that your session was outdated. This message indicates that multiple people are in the survey.
Why did I receive multiple survey links?
The Atlas Treatment Facility Survey collects information at the facility or clinic-level (i.e., by physical location). If you are the primary contact for multiple facilities, you will receive individual links to complete the survey for each of these physical sites. We ask that you submit the survey for all of your sites individually so that we can include this information in each facility profile on Treatment Atlas. Please contact us at Atlas@shatterproof.org if you have any questions.
How frequently are Atlas Treatment Facility Surveys collected from providers?
Shatterproof collects survey data from providers every two years to ensure the information displayed on Treatment Atlas remains as current as possible. Treatment facilities are asked to verify and update their information once every two years. If the information is not updated within this window, treatment information will be removed from your facility profile.
Where can I get a hard copy of the Atlas Treatment Facility Survey?
A hard copy of the TFS is available here.
I submitted a survey during the last Open Enrollment period. Do I need to submit a survey again?
All facilities, including those that submitted survey responses during the previous Open Enrollment period, are asked to submit the survey once every two years. We will send you an email with your survey link around two years since your last submission. Otherwise, you are welcome to submit updates anytime, particularly if your treatment services change. Simply email us at Atlas@shatterproof.org to get your survey link.
If you do not submit the survey, your facility or clinic will still be displayed on Treatment Atlas, but your facility profile will indicate that data is not available and any previous survey data displayed in your facility profile will be removed.
What happens if I do not submit responses to the Atlas Treatment Facility Survey?
If you do not submit the survey, your facility or clinic will still be displayed on Treatment Atlas, but your facility profile will indicate that treatment data is not available.
How can I request my survey link(s) be resent?
We are happy to resend the Atlas Treatment Facility Survey links upon request. Please email us at Atlas@shatterproof.org.
As a reminder, the Atlas Treatment Facility Survey invitation emails should have been sent to the primary contact for your site from Atlas@shatterproof.org.
Who do I contact if I have questions or need help completing the survey?
If you have questions or need help completing the survey, please reach out to the Treatment Atlas Team at Atlas@shatterproof.org. We will gladly assist you!
Survey Completion
Below, we provide guidance on how to complete the Atlas Treatment Facility Survey. If you have any additional questions or need assistance, please contact us at Atlas@shatterproof.org.
Do I need to respond to all questions in the survey?
Some questions in the survey are required, which means these questions must have a response before you can submit the survey. An asterisk (e.g., *) is used to indicate that the question requires a response. You will not be able to advance to the next page of the survey without answering all required fields on a page. You need to click the “Save & Next” button at the bottom of the page for your responses to save, so if you are unsure about an item and wish to move on in the survey for now, please fill in the answer you feel is most appropriate. You can always change the response prior to submission by using the “Table of Contents” or selecting the “Back” button and returning to the section and question. We encourage you to provide a response to all questions in the survey, as this will allow us to display comprehensive information about your treatment facility on TreatmentAtlas.org.

Why do some survey questions already have responses?
Some questions in the survey are “prepopulated,” which means they already include a response or selection(s) based upon the information you submitted to us in previous Atlas Treatment Facility Survey(s). Prepopulation is intended to make it easier for you to complete the survey. You may change any survey responses that are prepopulated by selecting or unselecting response options. We strongly recommend that you review all survey questions and associated response options in their entirety, even if a question is prepopulated, because a new response option may have been added. Note that your survey will only be prepopulated if you submitted survey responses to Atlas during previous Open Enrollment periods.

I responded to a question but am receiving an error message. How do I fix this?
Sometimes response selections have specific requirements that, if not completed, will prevent you from advancing to the next page. For example, if you select “Other” but do not provide a text response to specify what “Other” means, you will not be able to advance to the next page. You will see a warning at the top of the question(s) in red which tells you that the question is required. To advance, you should specify what “Other” means in the text field or unselect the “Other” response option.

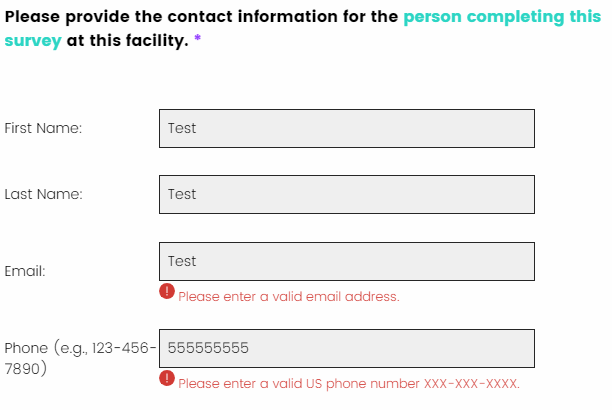
I entered an email address or a phone number and received an error message. How do I correct this?
There are several items that require specific formatting when providing a response. For example, an email address must contain an “@” symbol, and phone numbers must be entered with dashes (e.g., “123-456-7890”). In general, these rules are specified in the question text.
If a response is not entered correctly, an error message will appear directly below the survey response field, with specific guidance on how to update the response so that you can advance to the next page. To enter a valid email address, please submit in the following format: example@home.com.
How do I change a response to a survey question?
Your responses are not final until you attest and submit your survey. When in the process of completing the survey, you may change your responses, even in previous survey sections. To change a response, you can use the “Table of Contents” or the “Back” button to return to a specific section and survey question and either unselect a check-all-that-apply option by clicking it once more OR select a different response for questions that specify you choose only one response.


Why do the questions displayed in the survey change based upon my responses?
Many items in the survey use display logic. Display logic is used to personalize your survey experience by ensuring you are only asked survey questions that are relevant to you. For example, the question “Please identify the payment sources accepted at this facility” in Section ‘Site Details’ asks you to identify all payment sources your facility accepts.

Upon answering this question, you are asked to click “Save & Next”. The next survey question only displays choices that align with what you previously selected in response to “Please identify the payment sources accepted at this facility.”
If you realize you meant to select other response options, such as “Medicaid” simply click the “Back” button to change your responses to the previous survey question, and then click “Save & Next” again. Your survey will automatically update to reflect this change.
I am responding to a check-all-that-apply question and am unable to select more than one response option. How do I proceed?
Occasionally, you may note that an item allows you to “check-all-that-apply”. Generally, these items allow you to select more than one option. However, there may be one or more response options within an item which – if selected – prevent you from selecting any other response.
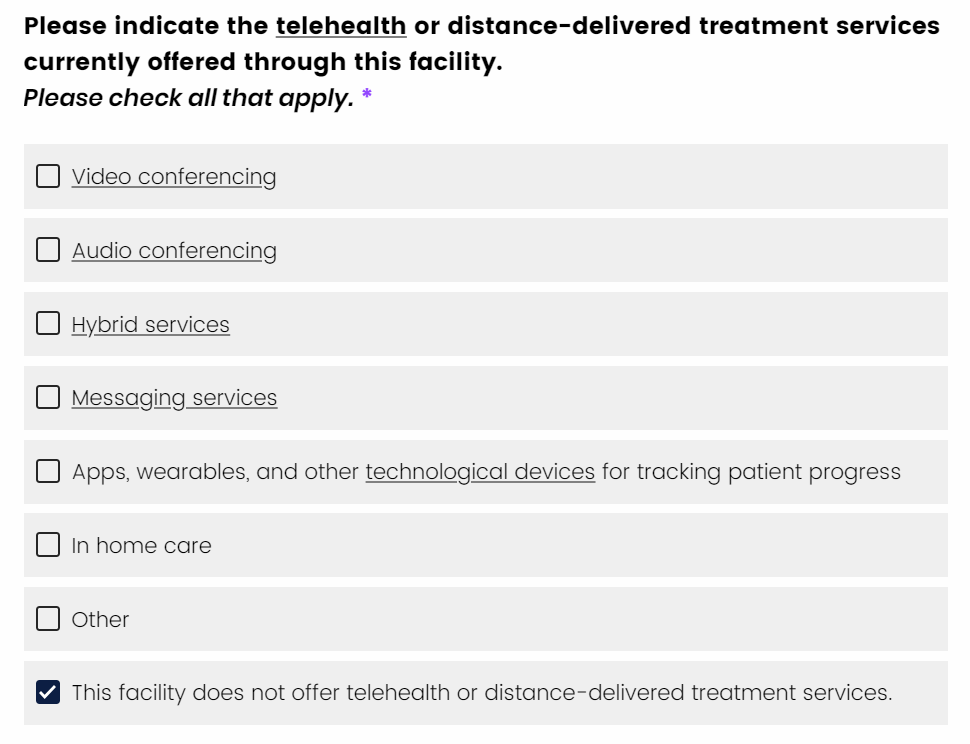
You'll notice that selecting a response option like “This facility does not offer telehealth or distance-delivered treatment services” is an “exclusive select,” meaning that if you do not offer that service, none of the other selection options can apply.
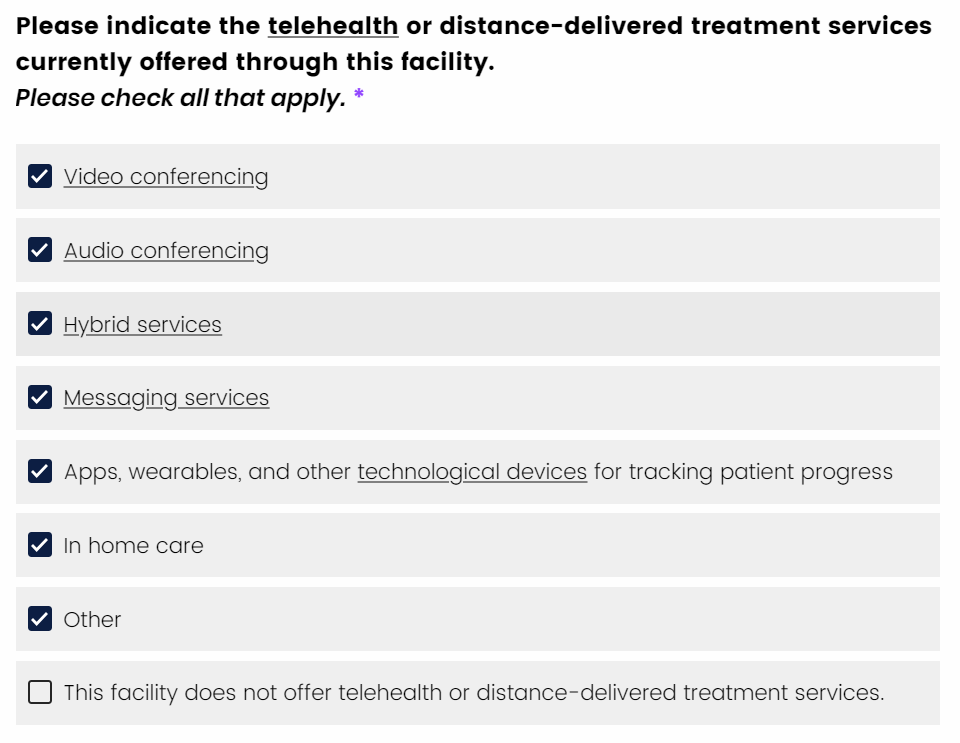
If you want to change a selection, you can select any option and the “exclusive select” option will be de-selected.
How do I upload documents or other materials in response to certain survey questions?
Throughout the survey there are fields which allow you to upload documents to support your submission. These fields allow one file that is up to 50MB in size to be uploaded. If you need to provide more files than allotted, we advise merging one or more files together into a ZIP file before uploading them as a single file. Upload items can support the following file types: PDF, Document (DOC, DOCX, TXT, ODT), Spreadsheet (CSV, XLS, XLSX, ODS), or Graphic (JPG, PNG, GIF). For security reasons, executable files (such as those ending in .exe) are not permitted.
Why are some words in the survey questions or response options underlined?
Throughout the survey, you will have access to an assortment of tooltips (i.e., pop-ups of informational text intended to support you in completing survey questions). When completing the survey on a web browser, tooltips will appear as underlined hyperlinks. You can hover your mouse over underlined text to see the tooltip. For example, the following question provides a definition for “electronic health record (EHR)” which can be viewed by moving your mouse over the underlined words.

If using a tablet or mobile device, the tooltip will be visible as an underlined hyperlink, but it will not be accessible. A comprehensive list of terms with definitions can be found in our Glossary below and you can also refer to the Atlas Treatment Facility Survey Reference Copy to see the tooltips.
I have entered the facilities open and closed times, but I do not see the “Next & Save” button to proceed to the next question.
The open and closed times need to be entered in the HH:MM AM/PM format. If you do not enter it in this format, you will not be allowed to proceed to the next question. If you enter only a number, the number will remain in red until the correct format is entered, this shows that there is an error in your response.
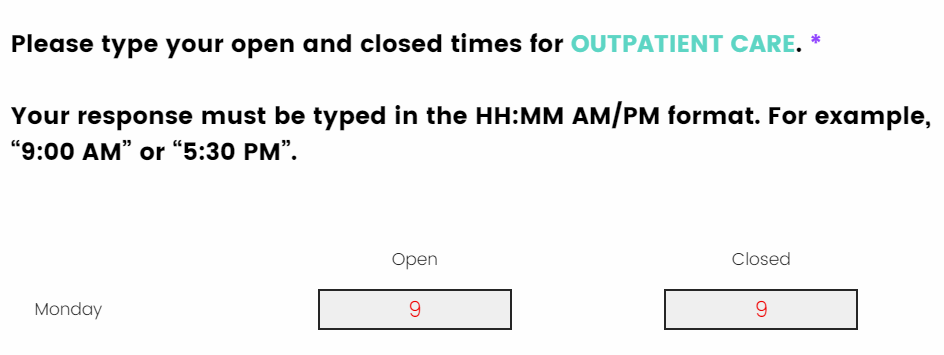
Entering a number followed by an AM/PM is an invalid entry. The “Next & Save” button will not appear until the correct format is entered.
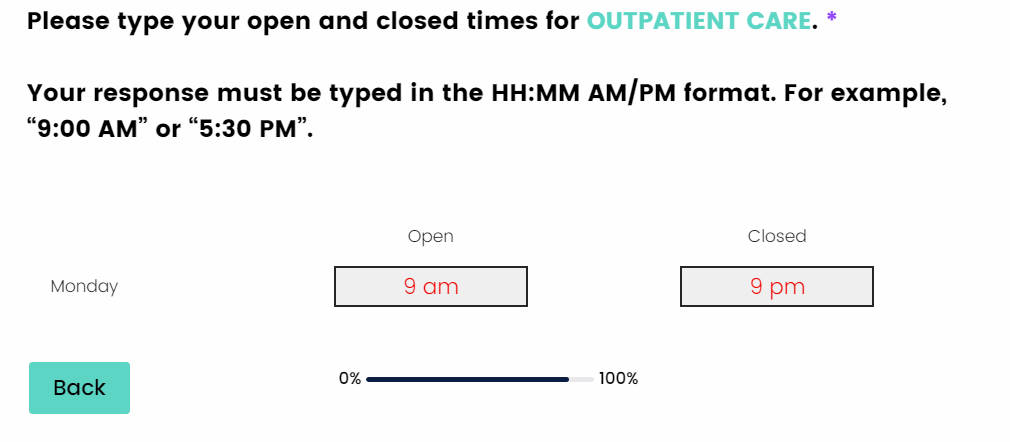
Here is what the correct format should look like.
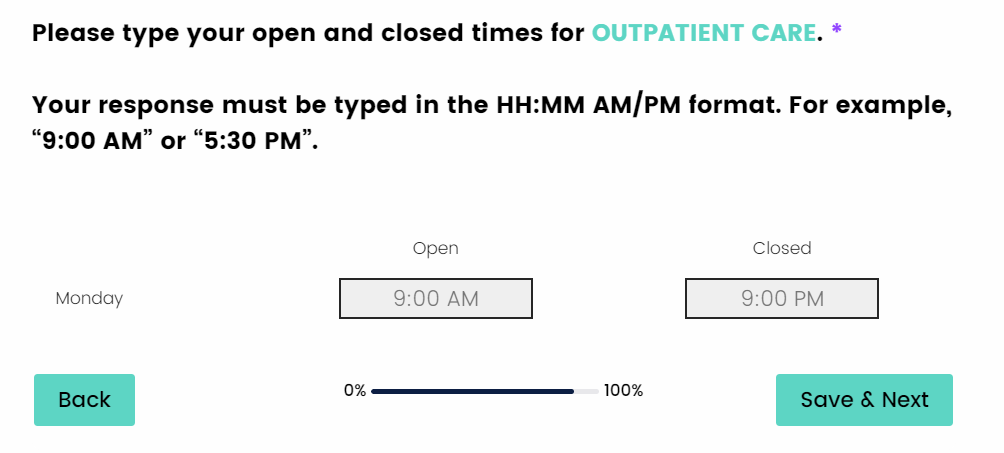
If you have any questions, please email us at Atlas@shatterproof.org.
How can I review all of my survey responses?
Before attesting to the accuracy of your responses and submitting your survey, we encourage you to engage in a full submission review. You can use the “Table of Contents” to navigate to each section to review your responses. You may also use the “Back” button to go back to a question and change any of your responses. Please note, if using the “Table of Contents” to navigate to a section, you may also need to use the “Back” button to get to the start of the section, as the “Table of Contents” may take you to the last position you were at within that section. We include links to each section of the survey on the very last page before you submit for convenience.


How do I complete attestation for my survey responses?
At the end of the survey, you will be asked to attest to the accuracy of your survey responses. To attest, you are required to have a member of your facility’s senior leadership review the survey responses you provided and complete the attestation form. This form requests that the senior leader click on the “I have read the information on this page and attest to the data submitted as accurate”. This indicates that they affirm the provided responses. They will also need to provide their name, title, organization, phone number, and email address. We will not share any contact information externally.

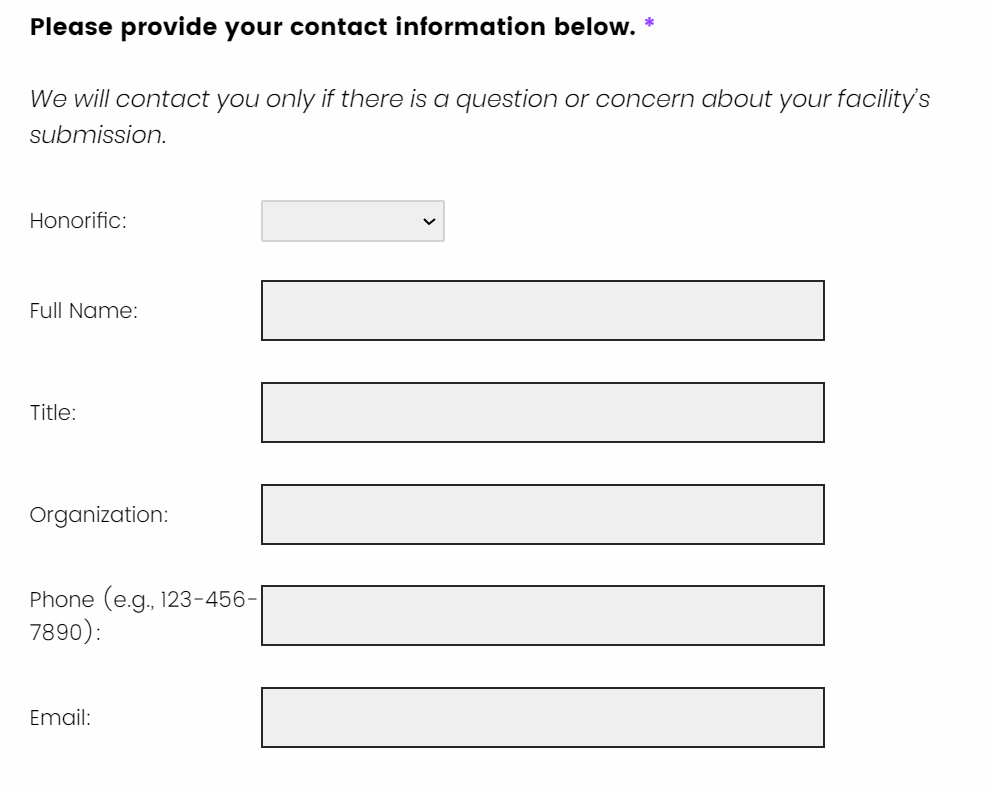
How do I submit my survey?
Your survey can be submitted only after it is attested. Once you have completed the attestation information and click on “Save & Next” you will be asked “Are you ready to submit?”.
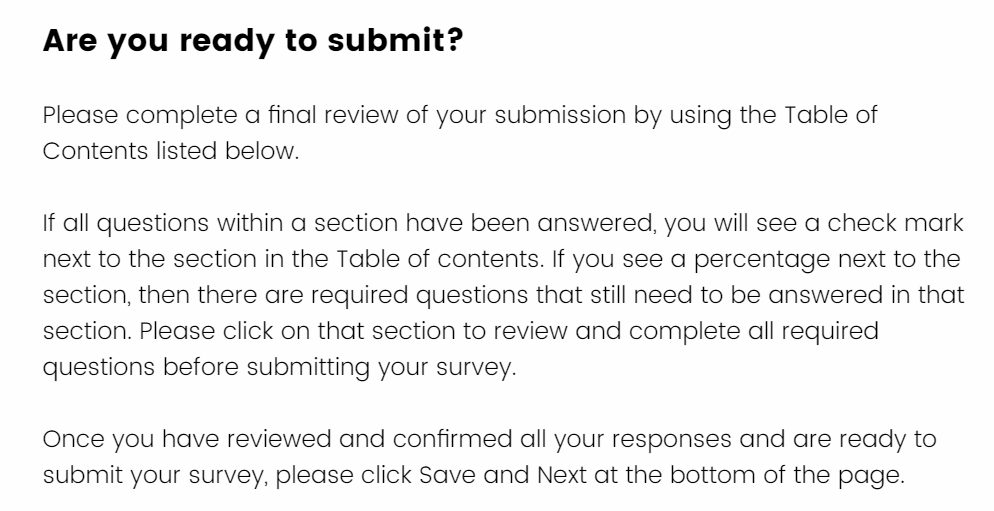
The Table of Contents that appears on this page will allow you to go back to any section of the survey to review your responses a final time. If you see a percentage next to a section in the “Table of Contents”, it means that the section is incomplete. We suggest that you click on that section to review and complete any required questions before submitting your survey.

If all questions and sections are complete you will see a check mark next to all the sections in the Table of Contents.

By clicking the “Save & Next” button on the “Are you ready to submit” page, you are submitting your survey to Shatterproof. After you submit, you should see the screen below.
You should also receive a confirmation email. Please allow up to one hour for the email confirmation to arrive before contacting us.
I would like a pdf copy of my survey responses. Who should I contact?
If you would like a pdf copy of your submitted survey responses for your records, please email us at Atlas@shatterproof.org.
I submitted my survey but need to change a response. How should I proceed?
We encourage you to carefully review each response for accuracy prior to submitting. You can easily change any of your survey responses prior to submitting your survey.
If you submit your survey, and then realize a change is necessary, please email the Treatment Atlas team at Atlas@shatterproof.org.
Who should I contact if I am experiencing a technical issue with the survey?
If you are experiencing a technical issue with the survey, you can email the Treatment Atlas team at Atlas@shatterproof.org. Please provide a description of the problem, and, if relevant, attach screenshots to clarify the error you are experiencing within the survey. A member of our team will reach out to you within two business days. In the meantime, please review our technical best practices below:
- Please take the survey using a browser other than Internet Explorer (e.g., Firefox, Google Chrome, etc.)
- Confirm with your organizations ITS/technical team that your URL is accessible behind any firewalls.
- While this survey can be taken from a mobile device, the layout may be easier to read on a desktop.
How can I update the primary contact for a facility?
If you wish to update your contact information or update the primary contact for your facility, please email the Treatment Atlas team at Atlas@shatterproof.org. Please provide us with your updated contact information or with the contact information for your facility’s new primary contact. Following this update, the new primary contact should receive the Atlas Treatment Facility Survey email reminders that include your facility’s survey link(s). We are also able to store multiple contacts for a facility in our database if needed. All contacts will receive survey reminder emails and programmatic updates
Glossary
Please find definitions for commonly used survey terms below. These definitions are provided within the survey as tooltips.
Acamprosate: An FDA-approved medication for the treatment of alcohol use disorder. Brand name Campral®.
Adolescent Community Reinforcement Approach and Assertive Continuing Care: According to NIDA, this therapy “is another comprehensive substance [use disorder] treatment intervention that involves the adolescent and his or her family. It seeks to support the individual’s recovery by increasing family, social, and educational/vocational reinforcers."
Amphetamines/stimulants: Examples include MDMA, phenmetrazine, and other unspecified amines and related drugs.
Audio conferencing: Phone conferencing or web calls without video.
Brief Strategic Family Therapy: According to NIDA, this therapy “targets family interactions that are thought to maintain or exacerbate adolescent drug [use] and other co-occurring problem behaviors. Such problem behaviors include conduct problems at home and at school, oppositional behavior, delinquency, associating with antisocial peers, aggressive and violent behavior, and risky sexual behavior."
Buprenorphine implant: An FDA-approved medication for the treatment of opioid use disorder. Brand name Probuphine®.
Buprenorphine injection: An FDA-approved medication for the treatment of opioid use disorder. Brand name Sublocade®.
Buprenorphine with naloxone: An FDA-approved medication for the treatment of opioid use disorder. Brand names Suboxone®, Zubsolv®, Bunavail®, Cassipa®.
Buprenorphine without naloxone: An FDA-approved medication for the treatment of opioid use disorder. Brand name Subutex®.
Cognitive Behavioral Therapy (CBT): NIDA and NIAAA identify cognitive behavioral therapy (CBT) as an evidence-based therapy. According to NIDA, “[it] is based on the theory that in the development of maladaptive behavioral patterns like substance use, learning processes play a critical role."
Community Reinforcement Approach (CRA): NIDA identifies the community reinforcement approach as an evidence-based therapy. According to NIDA, it “uses a range of recreational, familial, social, and vocational reinforcers, along with material incentives, to make a non-drug-using lifestyle more rewarding than substance use."
Contingency Management/Motivational Incentives: NIDA and NIAAA identify contingency management as an evidence-based therapy. According to NIDA, it “involves giving patients or clients tangible rewards to reinforce positive behaviors such as abstinence.”
Disulfiram: An FDA-approved medication for the treatment of alcohol use disorder. Brand name Antabuse®.
Electronic health record (EHR): An electronic health record (EHR) is the systematized collection of patient or client electronically-stored health information in a digital format. These records can be shared across different health care settings through network-connected, enterprise-wide information systems or other information networks and exchanges. EHRs may include a range of data, such as clinical notes, assessment results, behavioral health treatment history, medical history, medication and allergies, treatment plans, record of services received, referrals and billing information. EHRs facilitate access to patient or client information for facility staff, which supports the delivery of quality care.
Family Behavior Therapy: NIDA identifies family behavior therapy as an evidence-based therapy. According to NIDA, it “is aimed at addressing not only substance use problems but other co-occurring problems as well, such as conduct disorders, child mistreatment, depression, family conflict, and unemployment."
Functional Family Therapy: According to NIDA, this therapy “is another treatment based on a family systems approach, in which an adolescent’s behavior problems are seen as being created or maintained by a family’s dysfunctional interaction patterns. FFT aims to reduce problem behaviors by improving communication, problem-solving, conflict resolution, and parenting skills."
Hallucinogens: Examples include LSD, DMT, STP, mescaline, peyote, and psilocybin.
High-intensity outpatient (HIOP) or partial hospitalization: Similar to ASAM Level 2.5, at least 20 per week of clinical services.
High-intensity residential: Similar to ASAM Level 3.5, clinically managed high-intensity residential services, at least 20 hours per week of clinical services.
Hospital inpatient detoxification or medically managed inpatient treatment: Similar to ASAM Levels 4, medically monitored or managed intensive inpatient withdrawal management.
Hospital inpatient treatment: Similar to ASAM Levels 4, medically monitored or managed intensive inpatient services.
Hybrid services: Any mix of in-person and virtual attendance for group and individual services.
Inhalants: Examples include chloroform, ether, gasoline, glue, nitrous oxide, and paint thinner.
Injectable long-acting Naltrexone: An FDA-approved medication for the treatment of alcohol and opioid use disorders. Brand name Vivitrol®.
Intensive outpatient (IOP) treatment: Similar to ASAM Level 2.1, at least 9 hours per week of clinical services.
Levels of care: The clinical intensity of the treatment program. According to the American Society of Addiction Medicine (ASAM), there are four main levels of care in addiction treatment: (1) Medically managed inpatient services, (2) Residential services, (3) Intensive outpatient services or high-intensity outpatient services, (4) Outpatient services.
Low-intensity residential: Similar to ASAM Level 3.1, clinically managed low-intensity residential services, at least 9 hours per week of clinical services.
Matrix Model: NIDA identifies the Matrix Model as an evidence-based therapy, in which “Patients or clients learn about issues critical to addiction and relapse, receive direction and support from a trained therapist, and become familiar with self-help programs,” according to NIDA.
Messaging services: Messaging can consist of any written communication shared with patients such as SMS, texts, email and/or messages through third-party applications and/or portals.
Mindfulness-based Relapse Prevention: NIAAA identifies mindfulness-based relapse prevention as an evidence-based therapy. According to NIAAA, it “combines CBT skill building approaches with mindfulness practices, which promote flexible rather than “autopilot” responses to physical and emotional triggers to drink.”
Motivational Enhancement Therapy: NIDA and NIAAA identify motivational enhancement therapy as an evidence-based therapy. According to NIDA, it “is a counseling approach that helps individuals resolve their ambivalence about engaging in treatment and stopping their drug use.”
MOU: Memorandum of Understanding.
Multidimensional Family Therapy: According to NIDA, this therapy “is an outpatient, family-based treatment for teenagers who [misuse] alcohol or other drugs. MDFT views adolescent drug use in terms of a network of influences (individual, family, peer, community) and suggests that reducing unwanted behavior and increasing desirable behavior occur in multiple ways in different settings. Treatment includes individual and family sessions held in the clinic, in the home, or with family members at the family court, school, or other community locations."
Multisystemic Therapy: According to NIDA, this therapy “addresses the factors associated with serious antisocial behavior in children and adolescents who [misuse] alcohol and other drugs. These factors include characteristics of the child or adolescent (e.g., favorable attitudes toward drug use), the family (poor discipline, family conflict, parental drug [use]), peers (positive attitudes toward drug use), school (dropout, poor performance), and neighborhood."
Office-based provider: A general healthcare practice, such as a primary care office, that also offers substance use disorder treatment services. Examples include Office-based Outpatient Treatment (OBOT).
Opioids: Examples include heroin, fentanyl, and non-medical use of methadone, buprenorphine, codeine, hydrocodone, hydromorphone, meperidine, morphine, opium, oxycodone, pentazocine, propoxyphene, tramadol, and any other drug with morphine-like effects.
Oral Naltrexone: An FDA-approved medication for the treatment of alcohol and opioid use disorders. Brand names Depade®, Revia®.
OTC medications: Examples include aspirin, cough syrup, diphenhydramine and other antihistamines, sleep aids, and any other legally obtained non-prescription medication.
Outpatient detoxification or medically managed outpatient treatment: Similar to ASAM Levels 2.7 and 1.7, medically managed outpatient and intensive outpatient services.
Population-specific residential: Similar to ASAM Level 3.3, clinically managed population-specific high-intensity residential services.
Provides On-Site: On-site provision means that patients or clients are given prescriptions or dispensed medication on-site at this physical location.
Regular outpatient treatment: Similar to ASAM Levels 1.5 and 1.0, outpatient therapy or long-term remission monitoring.
Residential detoxification or medically managed residential treatment: Similar to ASAM Level 3.7, clinically managed residential withdrawal management
SAMHSA-certified Opioid Treatment Program (OTP): According to SAMHSA and CMS, Opioid Treatment Programs (OTPs) provide medication for addiction treatment to people diagnosed with an opioid use disorder. OTPs must be certified by the Substance Abuse and Mental Health Services Administration (SAMHSA) and accredited by an independent, SAMHSA-approved accrediting body.
Sedatives/hypnotics: Examples include mobarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, ethchlorvynol, glutethimide, and methaqualone.
Recovery Support Service: We consider your facility to be offering a service if it provides it on-site to interested patients or clients or if it actively engages or supports patients or clients in obtaining the service off-site. Direct referral or off-site access could be characterized by a formal agreement with an entity that provides a service (e.g., a childcare provider). The important distinction is that your facility actively supports and/or encourages participation in these services. A passive action, such as giving patients or clients a list of peer-support groups, is not sufficient.
Translation Services: Include clinical fluency, interpreting services, as well as facility generated educational and or administrative materials designed to relay information to non-English speakers.
Standardized assessments: Standardized assessments are tools or instruments that are developed with consistent predetermined protocols for administration, use, and analysis. Such assessments are administered to collect and understand information in a uniform manner.
State-financed health insurance: State-financed health insurance programs are programs created, sponsored, and/or funded (all or in part) by a state, county or local government and may provide: Medical care or financial assistance for medical care, health insurance, prescription assistance, medical supplies, medical equipment, disease screening, respite care, or other medical assistance.
Substance use disorder treatment: Treatment for substance use disorder is defined as any clinical practice designed to deliver withdrawal management (also known as detox), or services that focus on initiating and maintaining an individual’s recovery from substance misuse and preventing relapse.
Substance use disorder treatment facility: Facilities identified as specifically offering withdrawal management (also known as “detox”) or addiction treatment services that focus on initiating and maintaining an individual’s recovery from substance misuse and preventing relapse.
Technological devices: Certain technological devices may be used to support distance-delivered treatment. Examples include wristband sensors.
Telehealth: According to HHS, telehealth is defined as the use of telecommunication technologies (e.g., video conferencing, texting, smartphone applications) and electronic information to provide care in place of having patients or clients travel to the provider.
Tranquilizers: Examples include alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, flunitrazepam, flurazepam, halazepam, lorazepam, oxazepam, prazepam, temazepam, triazolam, and meprobamate.
Twelve-Step Facilitation Therapy (TSF): NIDA and NIAAA identify Twelve-Step Facilitation as an evidence-based therapy. According to NIDA, it “is an active engagement strategy designed to increase the likelihood of [an individual with substance use disorder] becoming affiliated with and actively involved in 12-step self-help groups, thereby promoting abstinence.”
Video conferencing: Certain technologies may be used to support distance-delivered treatment. Examples of video conferencing include Zoom and Skype.